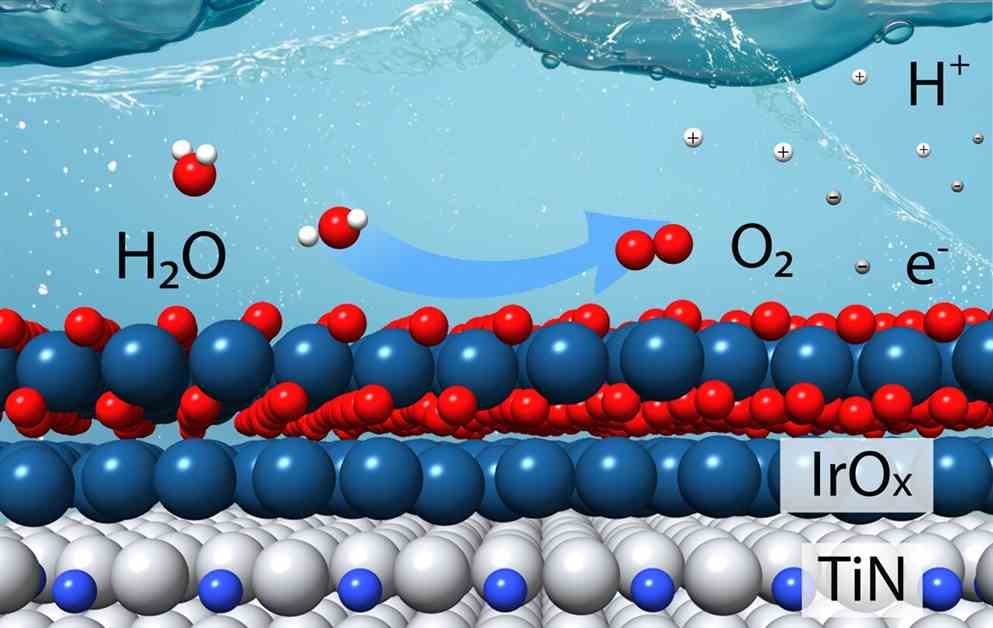
Scientists at the U.S. Department of Energy’s Brookhaven National Laboratory and Columbia University have successfully developed a new catalyst for the oxygen evolution reaction in water-splitting. This breakthrough could potentially lead to more efficient production of hydrogen as a clean fuel source.
Hydrogen is considered a promising fuel for reducing greenhouse gases, especially when produced by splitting water molecules using renewable energy sources. However, the process of breaking water into hydrogen and oxygen is complex and requires catalysts to facilitate the electrochemical reactions.
The new catalyst, designed based on theoretical calculations, aims to minimize the use of iridium, an expensive metal commonly used in catalytic materials. The team’s laboratory tests confirmed that the catalyst outperformed commercial iridium catalysts, requiring four times less iridium to produce hydrogen at the same rate.
The study highlights the importance of theoretical calculations in designing practical catalysts for real-world applications. Scaling up production remains a challenge, as current methods only allow for the production of small amounts of the catalyst.
By reducing the amount of iridium needed for the oxygen evolution reaction, the new catalyst offers a more cost-effective solution for water-splitting applications. Further research is needed to optimize the catalyst’s consistency in powder form for industrial use.
The successful development of this efficient water-splitting catalyst brings us one step closer to producing large quantities of green hydrogen and advancing towards a more sustainable energy future.