Researchers have made significant progress in understanding the body’s quality control system for protein folding, which could lead to targeted treatments for various diseases. The discovery of a critical “hot spot” where this process occurs sheds light on how misfolded proteins are identified and either corrected or destroyed.
The intricate process of protein folding is crucial for maintaining cellular functions, with misfolding leading to serious diseases like emphysema, cystic fibrosis, and Alzheimer’s disease. While our bodies have a quality-control system to address misfolded proteins, the exact mechanisms involved have been a mystery until now.
A team of researchers at the University of Massachusetts Amherst recently published their findings in the Proceedings of the National Academy of Sciences, unveiling the key players in the quality-control process. The enzyme UGGT and the partner protein Sep15 play pivotal roles in identifying misfolded proteins and determining their fate.
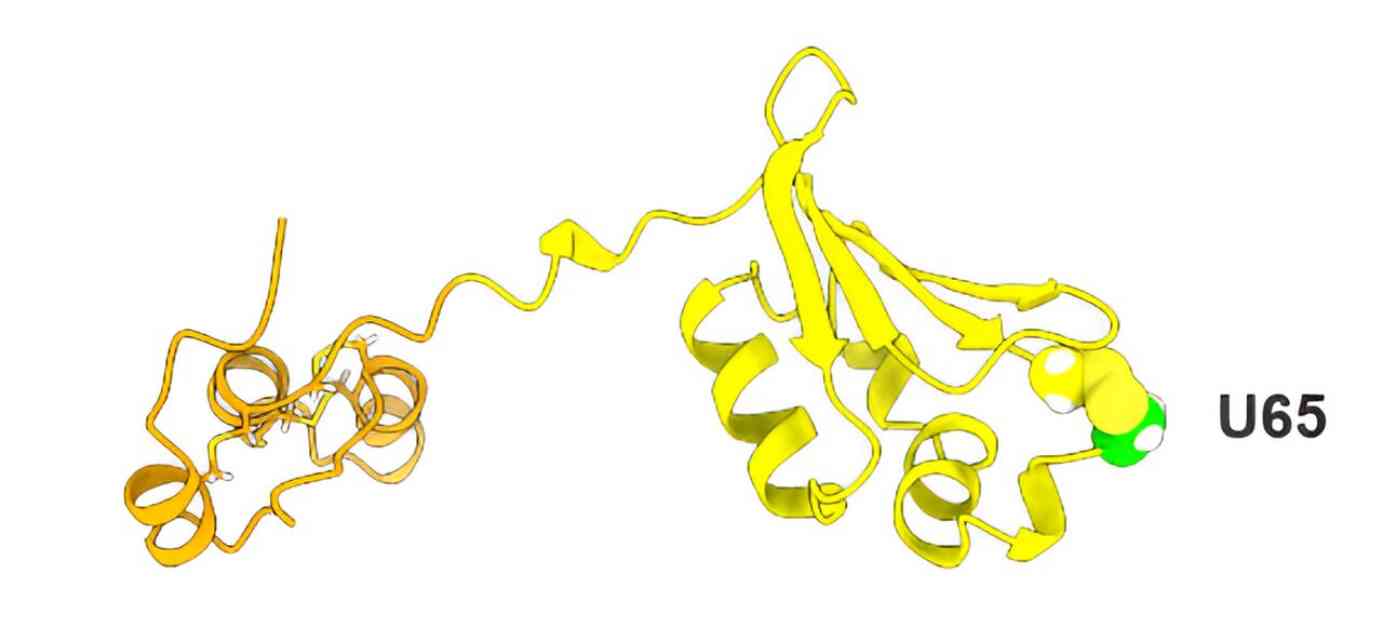
UGGT acts as a “gatekeeper” by reading carbohydrate tags on proteins to assess their folding status. However, the presence of Sep15, a selenoprotein, alongside UGGT has puzzled scientists for years. Through the use of an AI model called AlphaFold2, the researchers predicted that Sep15 forms a complex helical structure that interacts with UGGT like a catcher’s mitt, facilitating the recognition of misfolded proteins.
Lead-author Rob Williams emphasized the importance of Sep15 in the quality-control process, stating that it serves as the key to understanding how misfolded proteins are managed. By disrupting the binding between UGGT and Sep15 in an experiment, the researchers confirmed the critical role of Sep15 in facilitating protein folding.
The complexity of proteins in the body presents challenges in maintaining proper folding, as misfolded proteins can have severe consequences if not addressed. The team’s groundbreaking research not only sheds light on the fundamental mechanisms of protein quality control but also paves the way for the development of targeted therapies that focus on the Sep15/UGGT interface.
Subheadings:
Understanding Protein Quality Control
Proteins are essential molecules that carry out various functions in the body, from structural support to enzymatic activity. The process of protein folding, where a chain of amino acids adopts a specific three-dimensional structure, is critical for their proper function. However, misfolded proteins can lead to dysfunctional cellular processes and contribute to the development of diseases.
The endoplasmic reticulum (ER) serves as the cellular protein factory, responsible for synthesizing thousands of different proteins. Within the ER, proteins undergo a complex folding process that ensures their correct conformation before they are transported to their intended locations within or outside the cell. This quality-control mechanism is crucial for maintaining cellular homeostasis and preventing the accumulation of misfolded proteins.
The Role of UGGT and Sep15 in Protein Folding
UGGT, an enzyme within the ER, plays a crucial role in identifying misfolded proteins by reading carbohydrate tags called N-glycans. These tags provide information about the folding status of the protein, allowing UGGT to determine whether additional folding assistance is needed or if the protein should be targeted for degradation.
Sep15, a selenoprotein that partners with UGGT, was previously thought to be a mysterious bystander in the quality-control process. However, the researchers’ discovery of Sep15’s intricate structure and its interaction with UGGT revealed its essential role in facilitating protein folding within the ER.
Through advanced computational modeling using AlphaFold2, the researchers predicted that Sep15 forms a complex helical shape that complements the binding site on UGGT, resembling a catcher’s mitt. This structural insight provided a deeper understanding of how Sep15 guides UGGT in recognizing misfolded proteins and determining their fate.
Implications for Targeted Treatments
The identification of the Sep15/UGGT interface as a critical hotspot for protein quality control opens up new possibilities for targeted treatments aimed at correcting misfolded proteins. By disrupting the interaction between Sep15 and UGGT, researchers were able to observe the impact on the folding process, highlighting the potential for therapeutic interventions that modulate this interaction.
Dr. Daniel Hebert, a senior author of the study, emphasized the importance of further research to explore the dual role of Sep15 in either assisting misfolded proteins in correcting their shape or marking them for degradation. Understanding these mechanisms could lead to the development of novel drug therapies that target the Sep15/UGGT interface, offering new avenues for treating diseases associated with protein misfolding.
In conclusion, the groundbreaking research conducted by the team at the University of Massachusetts Amherst provides valuable insights into the body’s quality control regulator for protein folding. By unraveling the intricate interactions between UGGT and Sep15, researchers have laid the foundation for future studies and potential therapeutic interventions that could revolutionize the treatment of diseases caused by misfolded proteins.