# Unveiling Classical Arrhenius Chemical Kinetics Through Single-Molecule Reaction Analysis
The pinnacle goal of nanotechnology is the ability to program matter on the scale of individual atoms or molecules. Chemical reactions, whether desirable (such as in pharmaceutical manufacturing) or undesirable (like the breaking of DNA strands below a certain threshold), are mediated by charge transfer processes that occur on a single-molecule scale. A scanning tunneling microscope (STM) plays a crucial role in initiating and probing these processes in atomically controlled environments.
## The Role of STM in Chemical Kinetics
The STM tip is now routinely used to control the arrangement of individual atoms and molecules by leveraging the mechanical interaction between the tip and the target adsorbate. By employing the electric field in the gap between the tip and the sample, researchers have achieved control over the yield of chemical bond formation and the configurational isomerization of molecules. Moreover, the STM tunneling current can enhance specificity and control over a broader range of molecular reaction outcomes through charge capture or charge-state manipulation.
## Single-Molecule Manipulation and Reaction Outcomes
Researchers have demonstrated on-demand configurational switching of weakly-bound, cryogenically-stabilized single molecules in various systems. Notably, tip-controlled molecular motors, driven by intramolecular proton transfer and capable of transporting single molecules, have been successfully showcased. Control over the reaction outcomes is typically maintained by exciting different ionic resonances of a molecule, changing the charge injection location, or exciting different vibrations. In these scenarios, the reaction outcome is altered by the initial excitation mechanism, with subsequent dynamics evolving naturally from that point, resulting in a single possible reaction outcome.
## Investigating Energy Thresholds and Reaction Mechanisms
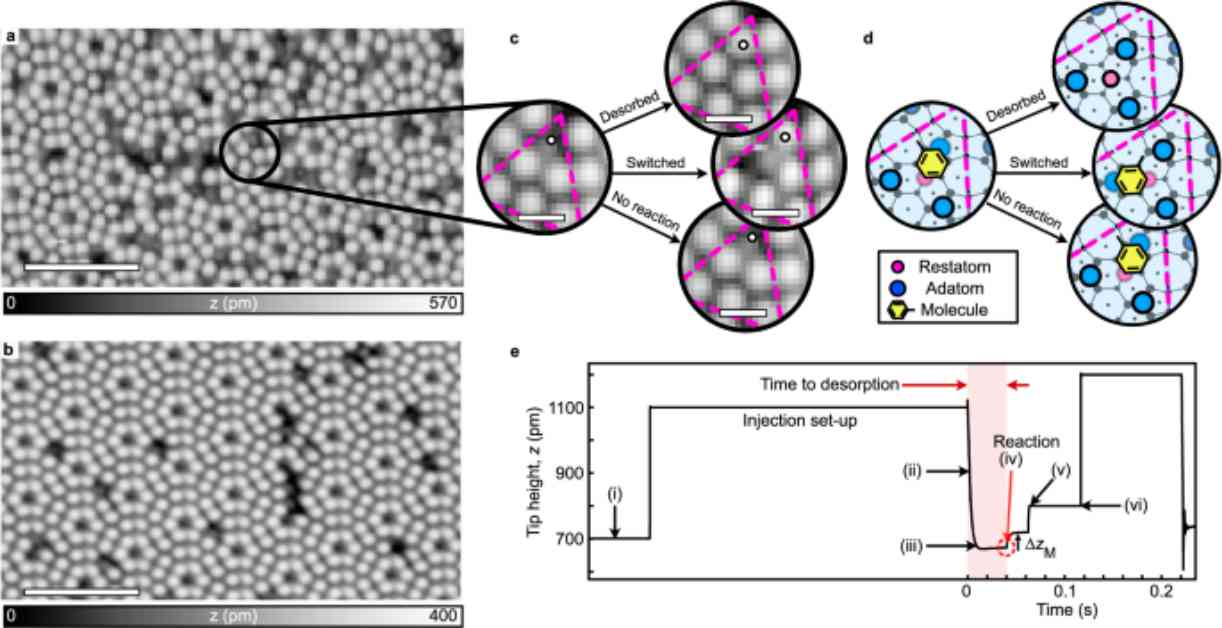
In a recent study, researchers delved into the realm of single-molecule reactions with multiple potential outcomes, focusing on the manipulation of single toluene molecules adsorbed onto a silicon surface. By increasing the energy of tunneling electrons above a specific threshold, the toluene molecules either switched to a neighboring adatom site or desorbed from the surface entirely. The challenge lies in the timescale of chemical reactions, which can take tens of femtoseconds to occur, while the resolution of STM electronics typically operates in milliseconds, necessitating inference of reaction mechanisms from measured probabilities rather than direct observation.
## Energy Dependence of Reaction Probabilities and Branching Ratios
The study further explored the energy dependence of the probability of desorption or switching for the single-molecule reactions, revealing a near-exponential increase in manipulation rates with the energy of injected electrons. Surprisingly, the branching ratio between desorption and switching exhibited a monotonic increase until a voltage threshold of 2.0 V, beyond which the branching ratio remained constant. This led to the development of a model based on classical chemical kinetics, suggesting an Arrhenius-type relationship for the reaction outcomes based on the energy of injected electrons and the thermal properties of the physisorbed state.
In conclusion, this research sheds light on classical Arrhenius chemical kinetics through the analysis of single-molecule reactions, showcasing the intricate interplay between energy thresholds, reaction mechanisms, and outcome probabilities in nanoscale chemical processes. By unraveling the underlying dynamics of these reactions, researchers are paving the way for precise control and manipulation of molecular systems at the atomic scale, offering new possibilities for future advancements in nanotechnology and molecular engineering.